Difference between revisions of "High-level Concepts v0.2"
Jump to navigation
Jump to search
m |
|||
| Line 46: | Line 46: | ||
***Enrolled population | ***Enrolled population | ||
***Eligible population | ***Eligible population | ||
| − | ***Screened population</font> | + | ***Screened population |
| + | </font> | ||
**<font style="color:blue">Excluded population | **<font style="color:blue">Excluded population | ||
***Excluded postrandomization population | ***Excluded postrandomization population | ||
***Not-randomized-population | ***Not-randomized-population | ||
***Not-enrolled-population | ***Not-enrolled-population | ||
| − | ***Not-eligible-population</font> | + | ***Not-eligible-population |
| + | </font> | ||
**<font style="color:blue">Analyzed-population | **<font style="color:blue">Analyzed-population | ||
***All subjects | ***All subjects | ||
***Study arm population | ***Study arm population | ||
***Crossover population | ***Crossover population | ||
| − | ***Subgroup population</font> | + | ***Subgroup population |
| + | </font> | ||
*Variables | *Variables | ||
**Independent variable | **Independent variable | ||
| Line 67: | Line 70: | ||
****Usual care | ****Usual care | ||
****Counseling | ****Counseling | ||
| − | ***Cointervention</font> | + | ***Cointervention |
| + | </font> | ||
**Dependent variable (responding variable) | **Dependent variable (responding variable) | ||
***Outcome | ***Outcome | ||
| Line 73: | Line 77: | ||
****Secondary outcome | ****Secondary outcome | ||
****Adverse event/Side effect | ****Adverse event/Side effect | ||
| − | ****Ancillary outcome</font> | + | ****Ancillary outcome |
| + | </font> | ||
*Digital and paper artifacts | *Digital and paper artifacts | ||
*Protocol | *Protocol | ||
**<font style="color:blue">Intended protocol | **<font style="color:blue">Intended protocol | ||
**Executed protocol | **Executed protocol | ||
| − | **Protocol change</font> | + | **Protocol change |
| + | </font> | ||
*Protocol application | *Protocol application | ||
**Assessments | **Assessments | ||
| Line 99: | Line 105: | ||
**<font style="color:blue">Treatment assignment | **<font style="color:blue">Treatment assignment | ||
**Blinding | **Blinding | ||
| − | **Follow-up</font> | + | **Follow-up |
| + | </font> | ||
*Measurement scale | *Measurement scale | ||
**Nominal | **Nominal | ||
| Line 109: | Line 116: | ||
---- | ---- | ||
| + | |||
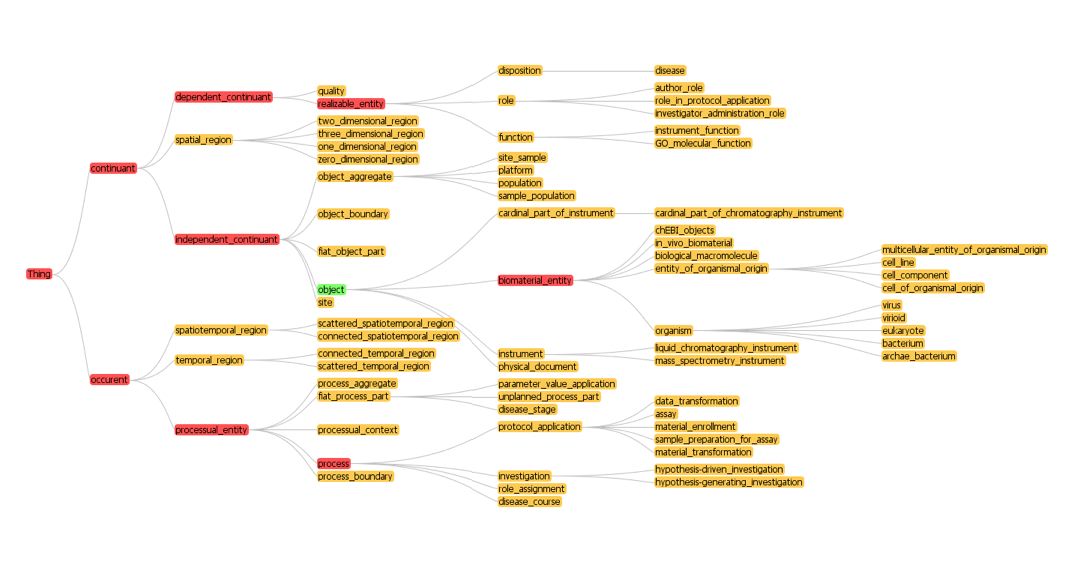
==High-Level Structure from BFO-OBI== | ==High-Level Structure from BFO-OBI== | ||
[[Image:High Level BFO_OBI.jpg]] | [[Image:High Level BFO_OBI.jpg]] | ||
Revision as of 11:09, 2 May 2007
Go back to CTO:Main Page
High-Level Concepts version 0.2
Initial High-level Concepts in black font
Additions from Simona in blue font
Additions from Richard in green font
- Events
- Periods
- Study phases
- Protocol phases
- Sequence of events
- Study designs
- Descriptive research – research in which the investigator attempts to describe a group of individuals based on a set of variable in order to document their characteristics
- Case study – description of one or more patients
- Developmental research – description of pattern of change over time
- Normative research – establishing normal values
- Qualitative research – gathering data through interview or observation
- Evaluation research – objectively assess a program or policy by describing the needs for the services or policy, often using surveys or questionaires
- Exploratory research
- Cohort or case-control studies – establish associations through epidemiological studies
- Methodological studies – establish reliability and validity of a new method
- Secondary analysis – exploring new relationships in old data
- Historical research – reconstructing the past through an assessment of archives or other records
- Experimental research
- Randomized clinical trial – controlled comparison of an experimental intervention allowing the assessment of the causes of outcomes
- Single-subject design
- Sequential clinical trial
- Evaluation research – assessment of the success of a program or policy
- Quasi-experimental research
- Meta-analysis – statistically combining findings from several different studies to obtain a summary analysis
- Randomized clinical trial – controlled comparison of an experimental intervention allowing the assessment of the causes of outcomes
- Descriptive research – research in which the investigator attempts to describe a group of individuals based on a set of variable in order to document their characteristics
- Research types
- Randomized Clinical Trial
- Methods
- Stakeholders
- Participants
- Investigators
- Monitors
- Study committee
- Sponsors
- Populations
- Recruited population
- Randomized population
- Enrolled population
- Eligible population
- Screened population
- Recruited population
- Excluded population
- Excluded postrandomization population
- Not-randomized-population
- Not-enrolled-population
- Not-eligible-population
- Excluded population
- Analyzed-population
- All subjects
- Study arm population
- Crossover population
- Subgroup population
- Analyzed-population
- Variables
- Independent variable
- Intervention
- Procedure
- Device implantation
- Drug treatment
- Placebo treatment
- Sham procedure
- Usual care
- Counseling
- Cointervention
- Intervention
- Independent variable
- Dependent variable (responding variable)
- Outcome
- Primary outcome
- Secondary outcome
- Adverse event/Side effect
- Ancillary outcome
- Outcome
- Dependent variable (responding variable)
- Digital and paper artifacts
- Protocol
- Intended protocol
- Executed protocol
- Protocol change
- Protocol application
- Assessments
- Experimental assays
- Observations
- Physical exam
- Interview
- Self-assessments
- Data analysis
- Data partitioning
- Data transformation
- Data pooling
- Data summarization
- Reliability
- Correlation
- Specimen processing
- Procurement
- Specimen partitioning/purification
- Specimen storage
- Treatment assignment
- Blinding
- Follow-up
- Assessments
- Measurement scale
- Nominal
- Ordinal
- Interval
- Ratio
- Participant characteristic (phenotype)
- Baseline characteristic